Introduction: Why Is Vor Biopharma Back on Investors’ Radars?
Vor Biopharma, once considered an overlooked small-cap biotech, is making a noticeable comeback in 2025. Following a challenging period marked by clinical setbacks and internal restructuring, the company is now regaining attention—and for good reason.
First, the company has made a major therapeutic pivot. With a strategic shift from oncology to autoimmune disorders—a segment full of unmet needs—Vor is targeting a space with significant upside potential and less competition.
Second, it secured a global licensing deal for telitacicept. This dual-target fusion protein, already approved in China for several autoimmune indications, gives Vor a commercially validated asset with global expansion prospects.
Finally, the market has responded emphatically. After a period of stagnation, the stock surged more than 500% in June 2025 following the deal announcement and a successful $175 million private placement that strengthened its cash position.
The Winning Trio: Cash, Pipeline & Leadership
This turnaround is supported by three strong pillars:
- Cash: After raising $175 million, Vor now has the financial flexibility to operate well into its next development milestones—a major de-risking factor for a clinical-stage biotech.
- Pipeline: With telitacicept as its new cornerstone, Vor’s R&D is now focused on a market with strong clinical and commercial potential.
- Leadership: The arrival of seasoned executives like Jean-Paul Kress, M.D. (former CEO at MorphoSys) and Sandy Mahatme (former CFO at Sarepta and a Moderna board member), brings industry credibility and executional expertise.
From Forgotten Small-Cap to Serious Contender?
This brings us to the central question for investors: Can Vor Biopharma transition from an overlooked small-cap to an emerging leader in immunology?
With strong cash reserves, a differentiated pipeline, and an experienced leadership team, the foundation is certainly in place. Furthermore, the early bullish sentiment from the market, as seen in the stock’s meteoric rise, confirms renewed investor interest. However, the company’s ability to execute on clinical trials and ultimately bring telitacicept to global markets will be the true test of this new strategic phase.
In short, Vor Biopharma enters a new chapter—one marked by regained credibility and high expectations. Every clinical milestone from here could be a game-changer.
The Market and Strategic Context
To understand the potential of Vor Biopharma’s new strategy, we first need to look at the market it is targeting: autoimmune disease therapies. This is one of the most dynamic and rapidly growing areas in the entire biopharmaceutical industry.
A Booming Market: The Rise of Targeted Therapies
The market for autoimmune treatments is expanding quickly. This is because the number of patients affected by these diseases continues to climb worldwide, and there is a soaring demand for more advanced and effective treatments. As a result, pharmaceutical companies are accelerating innovation, moving away from older, broad immunosuppressants and towards new, targeted “biologics” and precision therapies.
Furthermore, a significant “unmet medical need” remains. In simple terms, this means there are still many patients with rare or resistant autoimmune disorders for whom current treatments do not work well. This treatment gap is a major driver of market growth and creates lucrative opportunities for biotech companies with next-generation solutions.
Where Does Vor Biopharma Fit?
Vor Biopharma is now strategically positioned at the intersection of two major fields: blood cancers (onco-hematology) and autoimmune innovation.
While the company was historically focused on cancer therapies, its recent pivot to autoimmune diseases is a very smart move. By licensing telitacicept—a drug already approved for autoimmune conditions in China—the company has entered a high-potential market with a validated asset. Essentially, Vor is leveraging its expertise in immunology to bridge both oncology and the rapidly expanding autoimmune market.
Market Size and Competitive Landscape
The opportunity is massive. The global market for autoimmune disease treatments is estimated to be worth between $65 billion and $137 billion, depending on the scope.
However, this is also a very competitive field. Vor Biopharma is not competing with other small-cap biotechs alone. Its indirect competitors are some of the largest and most successful biopharma companies in the world, including:
| Market Segment | Estimated 2025 Size | Key Competitors in the Space |
| Autoimmune Therapeutics | $65B – $138B | AbbVie, Amgen, Roche, J&J, Novartis |
| Biopharmaceuticals (Broader) | ~$454 Billion | Pfizer, Merck, GSK, Sanofi, Biogen |
| Immune Cell Therapies | ~$4.3 Billion (High Growth) | Gilead, Novartis, bluebird bio |
In conclusion, Vor Biopharma’s pivot to autoimmunity is a strategic move into a massive and fast-growing market driven by significant unmet patient needs. Its success will now depend on its ability to execute its clinical development plan and compete against some of the biggest players in the industry.
A Closer Look at the Pipeline and Strategic Partnerships
A biotech company is only as good as its pipeline of potential new drugs. Vor Biopharma’s pipeline has been completely transformed, now featuring a powerful combination of a long-term innovative platform and a major, late-stage licensed drug.
Focus on the Pipeline: VOR33 and the Newly Licensed Telitacicept
Here are the key assets driving the company’s value:
- VOR33 (The Long-Term Innovation): This is Vor’s own engineered stem cell therapy, designed for patients with acute myeloid leukemia (AML), a type of blood cancer. In simple terms, VOR33 is designed to protect a patient’s healthy blood cells from a powerful cancer therapy, allowing the cancer to be targeted more effectively. It has received “Fast Track Designation” from the FDA and is currently in a Phase I/II clinical trial.
- Telitacicept (The New Cornerstone Asset): This is the company’s most significant recent addition. Vor secured an exclusive global license (outside of Greater China) from a company called RemeGen for this drug. Telitacicept is a treatment for several autoimmune diseases, including myasthenia gravis (gMG) and lupus (SLE). The key advantage is that this drug is already approved and sold in China, which means it has a proven track record of safety and efficacy. It is now in a global Phase 3 trial, which is the final stage before potential approval in the US and Europe.
The RemeGen Deal: A Major Strategic Move
The licensing deal for telitacicept is a massive, high-stakes partnership that instantly transformed Vor Biopharma.
Essentially, the deal gives Vor an advanced, de-risked drug that is much closer to generating revenue than its earlier-stage programs. The company is using the $175 million it recently raised to fund the final stage of clinical trials and prepare for a potential commercial launch. This move has given the company a clear, near-term path forward.
Telitacicept works by blocking two targets that are known to drive autoimmune diseases. This “dual-target” approach may make it more effective than other treatments that only block one target, especially for difficult-to-treat patients.
Pipeline & Key Milestones
This table summarizes the company’s main assets and their expected timelines for investors to watch.
| Program | Indication(s) | Stage (as of July 2025) | Key Milestones to Watch |
| VOR33 | Acute Myeloid Leukemia (AML) | Phase I/II | Enrollment is ongoing; initial results expected in 2026. |
| Telitacicept | Myasthenia Gravis, Lupus | Phase 3 (Global) | Topline results in 2026; potential market approval in 2027. |
| VORx / Next-Gen | Autoimmune / Blood Cancers | Preclinical | New drug candidates planned for 2025–2026. |
In conclusion, Vor’s pipeline is now a compelling mix. It combines the long-term, groundbreaking potential of its cell therapy platform (VOR33) with a near-term, high-potential commercial opportunity in the massive autoimmune market (telitacicept).
Fundraising & Financial Structure
A great pipeline is meaningless without the money to fund it. This is why Vor Biopharma’s recent $175 million private placement was such a critical and transformative event for the company.
The $175 Million Lifeline: Who and Why?
In late June 2025, Vor Biopharma announced it had raised $175 million from a syndicate of top-tier institutional biotech investors, including RA Capital Management, Forbion, and Venrock Healthcare Capital Partners. The fact that these well-respected, expert funds are backing the company is a major vote of confidence in its new strategy.
So, what is the money for? The funds are primarily being used to finance the acquisition and clinical development of the new autoimmune drug, telitacicept. In fact, $125 million of the total is specifically earmarked for the licensing deal with RemeGen. This clearly shows that the company is going “all-in” on its new focus on autoimmune diseases.
This new cash injection is expected to fund the company’s operations for at least the next 12 to 18 months, giving it a much longer “cash runway” to reach its next major clinical milestones.
The Trade-Off: Dilution vs. Strategic Gain
However, this fundraising comes at a cost for existing shareholders: dilution. To raise the money, the company issued 700 million “prefunded warrants,” which will eventually convert into new shares. In simple terms, this means that the total number of company shares will increase significantly, so each existing share will represent a smaller piece of the company.
This is a classic trade-off in the biotech industry. While dilution is a negative, it was a strategic necessity. This deal allowed the company to acquire a high-potential, late-stage drug and secure its financial future, which ultimately de-risks the investment and creates a much clearer path to potential success.
Before & After: A Snapshot of the Financial Transformation
This table shows the dramatic impact of the financing round on the company’s financial structure.
| Metric | Before Placement (est.) | After Placement (pro forma) |
| Market Cap | ~$260 Million | ~$435 Million* |
| Shares Outstanding | ~189 Million | ~889 Million (fully diluted**) |
| Net Cash Position | ~$60 Million | ~$235 Million (gross) |
| Cash Runway | < 6 months | 12–18 months |
Note: Final market cap will vary with the stock price.
*Approximate estimation; actual market cap post-dilution may vary with stock price reactions.
**Assuming all 700M warrants are exercised.
In conclusion, this financing round was a pivotal move. It has enabled Vor Biopharma’s strategic pivot into the massive autoimmune market, but it also required significant dilution that existing shareholders need to be aware of.
New Era of Leadership: The Team Behind the Turnaround
A company’s strategy is only as strong as the team executing it. In this regard, Vor Biopharma has recently made several high-profile executive appointments that send a clear and powerful message to the market: the company is preparing for the major leagues.
Key Appointments Signal a Shift to Maturity
The recent additions to the senior team underscore the company’s new focus on late-stage clinical development and international growth.
- Sandy Mahatme (Former Sarepta CFO): The appointment of a seasoned financial expert like Mr. Mahatme is a critical move. His experience in financing and scaling biotech companies is exactly what Vor needs as it navigates large, global deals and prepares for the significant costs of late-stage drug development.
- Dr. Qing Zuraw (Former RemeGen & FDA Clinical Development Expert): Dr. Zuraw’s hiring is a strategic masterstroke. Not only does she have over 25 years of experience in global clinical development at major companies like Janssen and Biogen, but she also personally led the successful late-stage development and approval of telitacicept in China. Therefore, there is arguably no one in the world better equipped to lead the charge to get this specific drug approved in the US and globally.
What This Means for Investors
These strategic hires are about more than just adding names to a roster; they reveal Vor Biopharma’s new strategy.
First, the company is preparing for execution. By bringing in leaders with proven track records of getting drugs approved and financed, Vor is signaling that it is transitioning from an early-stage research company to a mature, late-stage contender ready to run major clinical trials.
Furthermore, it sends a clear message of ambition. As CEO Jean-Paul Kress stated, Dr. Zuraw’s expertise will be “invaluable as we execute on our late-stage programs.” This new leadership gives the company the credibility and expertise needed to secure future partnerships and successfully bring its products to market.
In conclusion, the stock market has responded very positively to these appointments. This new, experienced leadership team is a core reason for the renewed investor confidence in Vor Biopharma’s potential to become a credible and successful player in the global immunology market.
A Look at the Financials: The Reality of a Clinical-Stage Biotech
While the new strategy, pipeline, and leadership team are exciting, it is critical for investors to understand the financial reality of a clinical-stage biotech company like Vor Biopharma. The company’s financial health is a major point of concern and highlights the high-risk nature of the investment.
Heavy and Recurring Losses
As Vor Biopharma does not yet have a product on the market, it is not profitable. In fact, the company continues to report substantial net losses as it invests heavily in research and development.
- The net loss for the first quarter of 2025 was $32.5 million.
- Over the past few years, annual net losses have averaged more than $100 million.
- As a result, the company’s cumulative deficit has now reached nearly $490 million.
Negative Cash Flow and the Need for Funding
This lack of profitability means the company has a negative cash flow. In simple terms, more cash is currently going out of the company to fund its operations than is coming in. This is why the recent $175 million private placement was so essential—it provided the necessary capital to continue its work. However, this cash runway is estimated to last for about 12 to 18 months, meaning the company will need to achieve positive clinical milestones to be able to raise more money in the future.
The Impact on Shareholders: Dilution
To fund its operations, Vor Biopharma has relied on selling new shares and warrants. This leads to significant shareholder dilution.
- The most recent financing deal included the issuance of 700 million prefunded warrants.
- The consequence for investors is that as more shares are created, each existing share represents a smaller percentage of the company. This is a common and major risk when investing in early-stage biotech companies.
In summary, Vor Biopharma has the classic financial profile of a high-risk, clinical-stage biotech company: heavy losses, negative cash flow, and a history of diluting shareholders to raise capital. This underscores the fact that any investment upside is entirely dependent on future success—whether through positive clinical trial results, a major partnership, or an acquisition. This high-risk profile is suitable only for investors with a high tolerance for volatility.
The Investment Thesis: Opportunities vs. Risks
For any clinical-stage biotech, the investment case is always a balance between future potential and present risks. Vor Biopharma is a perfect example of this high-stakes dynamic.
The Opportunities (The Bull Case)
First, the company now has a highly differentiated and de-risked pipeline. The newly licensed drug, telitacicept, is not just another early-stage idea. It is already approved and sold in China for several autoimmune diseases and is now in the final stage (Phase 3) of global clinical trials. This means a significant amount of the early scientific risk has already been removed. Furthermore, its unique “dual-target” mechanism offers the potential to be more effective than existing treatments.
Second, Vor Biopharma is well-funded to execute its plan. The recent $175 million in financing provides the company with a cash runway of 12 to 18 months. This is a critical advantage, as it gives the new leadership team the resources they need to fund the expensive late-stage clinical trials for telitacicept.
Finally, the timing is excellent. The company is pivoting into the autoimmune market at a time of rapid growth and innovation. There is a huge unmet need for better treatments for rare and complex autoimmune diseases, which is exactly where Vor is now focused.
The Risks to Watch (The Bear Case)
However, the risks are just as significant and must be understood.
First and foremost is the severe and persistent financial weakness. Vor Biopharma is a classic “cash-burning” biotech. The company is not profitable and has a long history of significant losses.
- Recurring Losses: The company reported a net loss of $32.5 million for the first quarter of 2025 alone and has a cumulative deficit of nearly $490 million.
- Negative Cash Flow: It consistently spends more cash on its operations than it brings in, making it entirely dependent on external funding to survive.
- This financial reality means that while the recent fundraising provides a temporary lifeline, the company’s underlying business does not generate its own money.
The second major risk is shareholder dilution. To fund its operations, the company has repeatedly sold new shares. The recent financing, for example, included 700 million new warrants. This means that the ownership stake of existing shareholders is significantly diluted, which can limit the potential upside of their investment.
Finally, there is always clinical trial risk. The company’s entire future is dependent on positive results from its pivotal Phase 3 trials. Any setback or failure in these trials would have a major negative impact on the company’s value, especially given its weak financial foundation.
In conclusion, Vor Biopharma’s new strategy and advanced drug provide a real and exciting opportunity. But at the same time, the classic clinical, financial, and competitive risks of the biotech industry remain very high. Success now depends entirely on flawless execution and clinical validation on a global scale.
Stock Market: Analysis and Outlook for Vor Biopharma (VOR)
After analyzing the company’s new strategy and its financial challenges, it’s essential to look at how the stock market has reacted and what to expect in the short term.
Price Evolution: A Spectacular Rebound
Vor Biopharma’s change in strategy has created an electric shock in its stock price.
- The performance: Since the announcement of the partnership with RemeGen and the $175 million fundraising in early June, the stock has jumped by more than 60%. It has moved from around $1.32 to a recent range of $2.02 – $2.36 (with a closing price of $2.12 on July 17, 2025).
- The volumes: Furthermore, trading volumes have literally exploded, with several sessions exceeding 10 million shares traded. This is a sign of strong renewed interest from investors and high speculation around the stock.
Market Sentiment: What Are the Analysts Saying?
This renewed interest is also visible among the financial analysts who cover the company.
- The consensus is clearly a “Buy,” confirmed by the 7 analysts who cover the stock.
- The average 12-month price target is around $3.77, which represents a potential upside of more than 82% from the current price.
Technical Analysis and Catalysts to Watch
From a technical perspective, most indicators are green, signaling a bullish trend. However, some indicators like the RSI are close to the “overbought” zone, which calls for some caution as volatility remains very high.
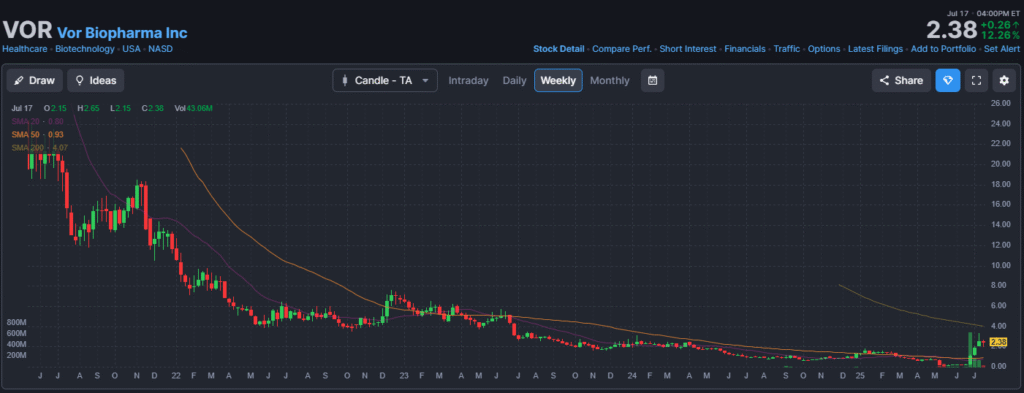
Looking ahead, the stock’s performance will be directly linked to achieving several key milestones:
- Clinical results for telitacicept: These are the most anticipated data points and will be absolutely decisive for the company’s strategy.
- Progress of the legacy pipeline: News on the Phase 1/2 trial for VOR33 will also be closely watched.
- New partnerships: Rumors or announcements of new collaborations could also serve as a catalyst.
In conclusion, after a period of correction, the stock is now in a very positive dynamic, driven by high speculative interest and favorable analyst opinions. The market is now waiting for Vor Biopharma to deliver on its promises. The next phase will, therefore, be dictated by the clinical results and the company’s ability to prove the value of its new pipeline.
Conclusion: The Investment Thesis for Vor Biopharma
So, is Vor Biopharma a high-risk speculative bet, or is a major re-rating of the stock already in progress?
Vor Biopharma today represents a classic high-risk, high-reward biotech investment that is currently undergoing a major strategic transformation. Following its pivot toward autoimmune diseases, a major global licensing deal, and a successful $175 million financing, the company is now signaling a new era of maturity and ambition to the market.
The market has already started to recognize this shift. For instance, the stock is up more than 60% since June 2025, trading volumes have surged, and Wall Street analysts have begun raising their price targets, with the consensus now implying a potential 80% upside.
Upcoming Catalysts to Watch
| Event | Estimated Timing |
| Q2 2025 Earnings Report | August 8, 2025 |
| Full Pipeline Presentation | Q4 2025 |
| Phase 3 Data for Telitacicept | Late 2025 – Early 2026 |
| New Drug Filings (Next-Gen Programs) | Q4 2025 – Q1 2026 |
Exporter vers Sheets
Bottom Line: Vor Biopharma is at a pivotal moment. It remains a speculative small-cap biotech, but it is showing clear signs of a potential re-rating. Armed with stronger financials, high-impact leadership, and a globally relevant autoimmune pipeline, the company is rapidly gaining momentum. Therefore, successful execution on its upcoming clinical milestones could make it a breakout candidate in the autoimmune space.

